Description
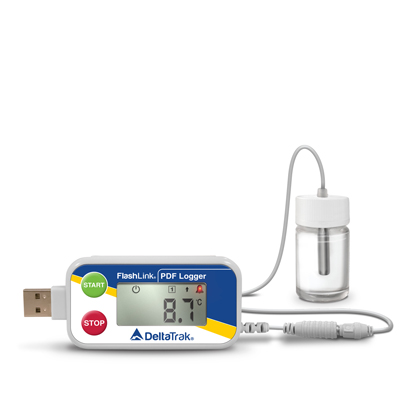
The FlashLink Certified Vaccine USB PDF Data Logger is pre-qualified by the World Health Organization (WHO) as a Temperature Monitoring Device, under their Performance Quality Safety code PQS E006/047, and is compatible with FDA, CDC and WHO guidelines. Certificates are available for ISO 17025 and NIST calibration. It’s specifically designed for monitoring temperature conditions inside storage units where vaccines and other temperature sensitive liquid medicines are kept.
This reusable logger features a large, easy-to-read LCD and an external probe sealed in a 5ml glycol bottle. The glycol prevents false alarms by buffering the sensor against sudden and expected temperature fluctuations during regular use, such as frequent door openings and normal cycling of the compressor within the refrigerator or freezer. The glycol also emulates temperature changes experienced by most common fluids in storage, to give more accurate readings.
The logger attaches to the outside of the storage door, while the sensor bottle is placed inside, allowing users to read current and minimum/maximum temperatures without opening the unit. A red LED alarm and low battery indicator are also visible on the display. This logger is compatible with FDA, CDC and WHO guidelines. Certificates are available for ISO 17025 and NIST calibration.
The FlashLink Certified Vaccine USB PDF Data Logger provides an accurate reading, programmable alarms and sampling intervals, and a USB connection to quickly and easily download temperature data. The FlashLink Certified Vaccine USB PDF Data Logger can be programmed to start automatically or with the option for a delayed start allowing the glycol bottle sufficient time to stabilize to the refrigerator or freezer temperature